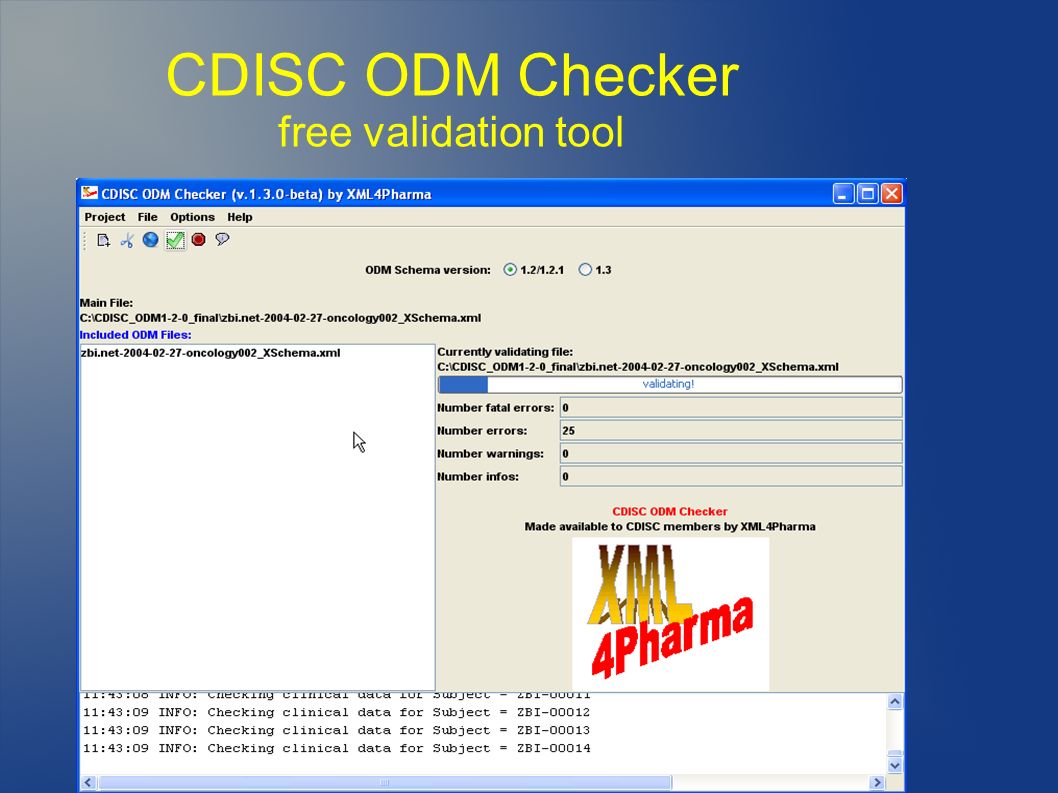
![PDF] Validating CDISC SDTM-Compliant Submission-Ready Clinical Datasets with an In-House SAS ® Macro-Based Solution | Semantic Scholar PDF] Validating CDISC SDTM-Compliant Submission-Ready Clinical Datasets with an In-House SAS ® Macro-Based Solution | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/69e25edb541540b03f8728c87c69e8a350ea7d3f/6-Figure1-1.png)
PDF] Validating CDISC SDTM-Compliant Submission-Ready Clinical Datasets with an In-House SAS ® Macro-Based Solution | Semantic Scholar

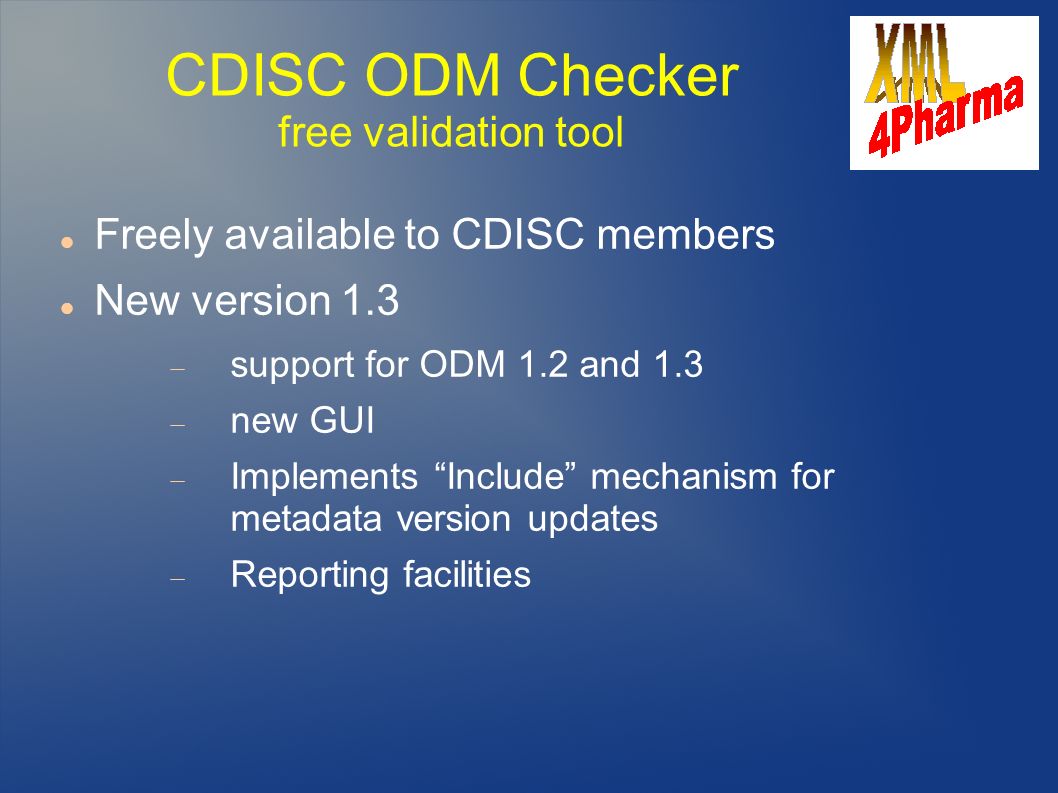
Sanofi-Aventis Experience Submitting SDTM & Janus Compliant Datasets* SDTM Validation Tools - Needs and Requirements - PDF Free Download
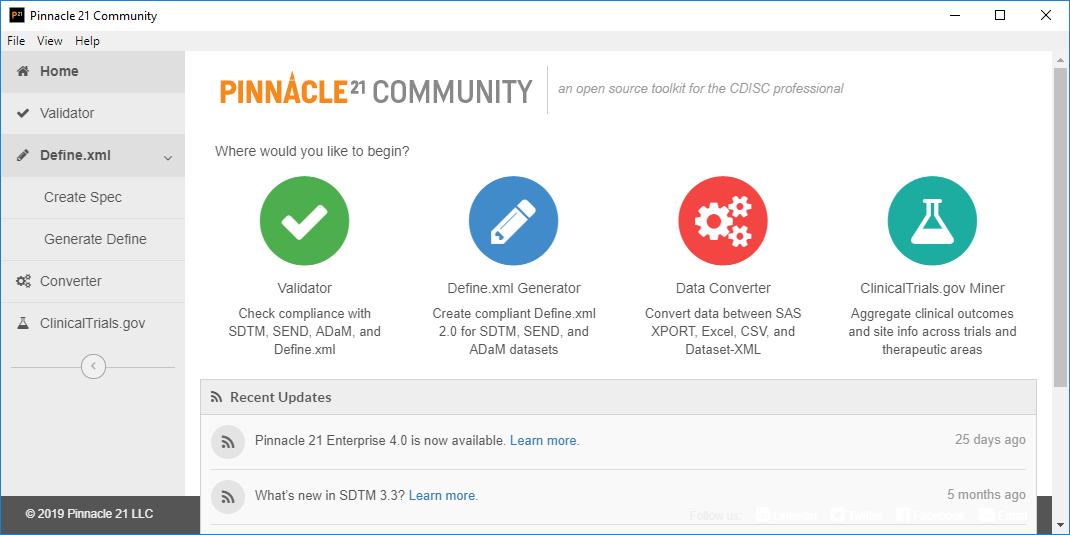
![PDF] In-Depth Review of Validation Tools to Check Compliance of CDISC SDTM-Ready Clinical Datasets | Semantic Scholar PDF] In-Depth Review of Validation Tools to Check Compliance of CDISC SDTM-Ready Clinical Datasets | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/4ea1ea0e99952efdcc712efee14e0d18e3b4208e/4-Figure3-1.png)
PDF] In-Depth Review of Validation Tools to Check Compliance of CDISC SDTM-Ready Clinical Datasets | Semantic Scholar
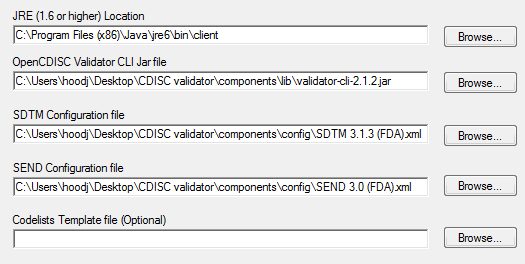
![PDF] In-Depth Review of Validation Tools to Check Compliance of CDISC SDTM-Ready Clinical Datasets | Semantic Scholar PDF] In-Depth Review of Validation Tools to Check Compliance of CDISC SDTM-Ready Clinical Datasets | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/4ea1ea0e99952efdcc712efee14e0d18e3b4208e/2-Figure1-1.png)
PDF] In-Depth Review of Validation Tools to Check Compliance of CDISC SDTM-Ready Clinical Datasets | Semantic Scholar
![PDF] In-Depth Review of Validation Tools to Check Compliance of CDISC SDTM-Ready Clinical Datasets | Semantic Scholar PDF] In-Depth Review of Validation Tools to Check Compliance of CDISC SDTM-Ready Clinical Datasets | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/4ea1ea0e99952efdcc712efee14e0d18e3b4208e/5-Figure4-1.png)
PDF] In-Depth Review of Validation Tools to Check Compliance of CDISC SDTM-Ready Clinical Datasets | Semantic Scholar
![PDF] In-Depth Review of Validation Tools to Check Compliance of CDISC SDTM-Ready Clinical Datasets | Semantic Scholar PDF] In-Depth Review of Validation Tools to Check Compliance of CDISC SDTM-Ready Clinical Datasets | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/4ea1ea0e99952efdcc712efee14e0d18e3b4208e/3-Figure2-1.png)

![CDISC® SDTM Conversion Made Easy with CDISC Express - [PDF Document] CDISC® SDTM Conversion Made Easy with CDISC Express - [PDF Document]](https://demo.fdocuments.in/img/378x509/reader024/reader/2021022513/568c0d5c1a28ab955a8c6f06/r-2.jpg)